Participant information sheet (intervention development study) – v3 (09-05-25)
Title of Project: Breathing Retraining for people with AsTHma and breathing pattern disorder (BREATH) – Intervention development study
Name of researcher: Adam Handley
You are being invited to take part in a research study to help us develop and test a new treatment for people who suffer with asthma. Before you decide, it is important for you to understand why the research is being done and what it will involve. This document gives you important information about the purpose, risks, and benefits of participating in the study. Please take time to read the following information carefully. If you have any questions, then feel free to contact the researcher whose details are given at the end of the document. We recommend taking 24 hours to decide whether or not you wish to take part. Your decision whether to take part in the study or not will have no bearing on your medical care.
What is the purpose of the study?
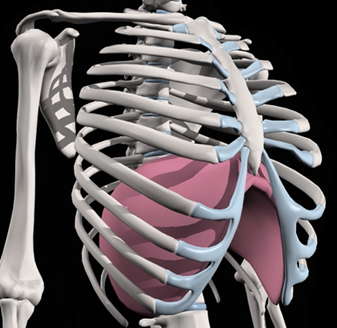
People with asthma can experience breathing difficulties for two reasons. The first is that tubes to the lungs become narrowed and swollen – this is treated using inhalers. The second reason is that the muscles around the lungs are not working properly. For example, the diaphragm is a big flat muscle under the lungs (see image). If this muscle isn’t working properly, then it limits air flow into the lungs, leading to feelings of breathlessness. The aim of this study is to develop and test a new treatment for people with asthma which will enable them to improve the way the muscles around their lungs work.
The new treatment will teach patients how to how to stand, move and breathe with less muscle tension. It will also teach patients to reduce stress and negative thoughts associated with breathing difficulties. Once people learn to breathe with less muscle tension, it can make breathing easier and may reduce symptoms of breathlessness during daily activities.
Why have I been invited to take part?
You have been invited because you have a diagnosis of asthma and are taking regular asthma medication. If you experience breathlessness, despite taking medication, then you may benefit from a new treatment for breathing pattern disorder.
Do I have to take part?
No, taking part is completely voluntary. If you are interested, contact the researcher (details at the end of this information sheet).
What will happen to me if I participate in this study?
If you are considering participation in this study, you will provide details to allow us to understand if you are eligible, such as height, weight and asthma symptoms via an online form or over the phone. If you completed the online form and you are not eligible, you will receive an email from the research team. If you are eligible, the research team will call you and you will sign a consent form. You will then be asked to complete a set of questionnaires which allow us to understand your symptoms and how breathing problems interfere with your life. You will be asked to complete these same questionnaires at the end of the study, after receiving the treatment.
If you are in group 1 (first five patients), you will be required to attend a workshop (at the University of Salford) before the start of the treatment. At the workshop (lasting 2-3 hours), we will present the key ideas behind the treatment and then ask you to suggest how we should design the treatment to make it acceptable to patients with asthma. You will then receive the treatment from a physiotherapist over a 7-week period (see description below). After the treatment, you will be required to attend a follow-up workshop (lasting 2-3 hours) which will allow us to understand how we can improve the treatment. At each workshop, we will record what is said and then summarise the key ideas in a document. The recordings will then be deleted. Your answers will be completely anonymised, and nobody will be able to link any answers back to you.
If you are in group 2 (second five patients), you will not need to attend a workshop and will receive the treatment (see description below) straight away. After the treatment, you will be interviewed (over phone or video conference) by another researcher (not the physiotherapist) about your experiences of the treatment. Note that if you are unfamiliar with video calls, we can help you to set this up. After it has finished, the researcher will listen to the recording and summarise your answers. Note that your answers will be completely anonymised, and nobody will be able to link any answers back to you.
To receive the treatment, you will be required to visit the University of Salford on between 5-8 separate occasions, each lasting approximately one hour. These visits will typically be 1 week apart. After each session, a researcher (not the physiotherapist) may ask you about any parts of the treatment your found difficult to understand. Note the first and last visit will last for 1 hour 30 minutes to allow us to perform an assessment of your breathing before the first and after the final treatment session. During the 30-minute assessment session, we will take several measurements. These measurements will include weight, height, hip/waist ratio, a measure of heart rate (using a chest strap) and of blood oxygenation (using a small finger sensor). We will also measure lung function by asking you to breathe into a tube that measures air flow. This assessment will require you breathe out as hard and fast as possible into the tube.
The final part of the assessment will be a measurement of how you breathe. This will require the researcher to place a set of 30-40 small stickers over your chest, stomach and back. To place these stickers, you will need to remove the clothing from your upper body. If you are female, you will wear a sports bra which we can provide if you don’t have one. After the stickers have been placed, you will stand and breathe normally in between two movement sensors which will measure your breathing pattern. These sensors identify the positions of each sticker and then use the data to calculate how the shape of your upper body changes as you breathe. This assessment should take about 1 minute, after which you will put a loose-fitting top back on. The researcher who will place the stickers will be female, and you can also request an additional chaperone if it would make you feel more comfortable.
The treatment
The physiotherapist will begin by explaining the how the muscles work which control breathing and how changes in the way muscles work can lead to breathlessness. They will also explain how breathlessness from changes in muscle control will feel different from true asthma (narrowing of the tubes in the lungs). You will then be taught how to consciously relax your stomach muscles using belly breathing and how to increase the motions of your ribs to make your rib cage increase in size. The next stage of the intervention is focused on teaching you to stand with less muscle tension. This is achieved using simple exercises which enable you to build awareness of patterns of muscle tension, particularly around your stomach, back, shoulders and neck.
Once you can stand with a relaxed posture, the physiotherapists will work with you to ensure that your ribs and diaphragm move together as you breathe in normal standing. The focus then shifts to everyday tasks which might cause you to feel breathless or stressed. To help you breathe more normally and relax, the physiotherapist will encourage you to imagine these situations and work with you, providing hands on guidance, to help you retain a good breathing pattern. Once you can do this, you should be able to maintain a normal breathing pattern and stay relaxed in situations which previously caused you to become breathless and/or stressed. If you want, this could include activities which require you to exert yourself, such as fast walking or cycling on an exercise bike.
To help you visualise the way in which you breathe, the physiotherapists may use special software to produce a visualisation of your breathing pattern on a computer screen. Note that this will require you to remove your top (and to wear a sports bra if you are female). As explained above, the physiotherapist will place small stickers on your upper body which the computer software tracks to measure breathing. Note that, while the breathing visualisation system does show a video, this data will not be stored and there will be no recording of you during this breathing visualisation.
As you progress through the treatment, you will gain a new experience of breathing which may feel strange at first but which you will get used to. You may also feel slight dizziness as your breathing improves but this will only last for a short amount of time. The physiotherapist may ask you to wear an oxygen saturation monitor (small sensor) on one of your fingers to monitor changes to your blood oxygen levels during the treatment. To help you understand many of the ideas which underlie the new treatment, animated instructional videos are used. These videos are watched through a special website which we will give you access to at the start of the treatment.
Once you have completed the study, we will write a letter to you GP confirming details of the treatment that you have received. In this letter, we will include a summary of the clinical notes that have been made throughout the course of the treatment. Note that the physiotherapist will assess your breathing at the start of the treatment. If they think that you have breathing difficulties that could be related to a medical condition for which you are not current receiving treatment, then they will write to your GP straight away and advise you to make an appointment with your GP. In this situation, you will not be able to continue with this study until you have been seen by your GP.
Expenses and payments?
Unfortunately, we are not able to pay you to receive the new treatment. However, we can cover reasonable travel expenses from within the Greater Manchester area, including costs of parking. You will also receive £20 per hour when you attend a workshop or have an interview after receiving the treatment.
What are the possible disadvantages and risks of taking part?
This is a very simple, straight forward study. The physiotherapist will be using techniques which are used in routine clinical practice, and these will be complemented with our breathing visualisation system which does not carry any risk. However, some people occasionally feel dizzy when receiving breathing retraining because of changes in their blood oxygen saturation level. We will monitor blood oxygenation and ensure that you sit or lie down if you feel dizzy.
What are the possible benefits of taking part?
You will receive 5-8 sessions of the new treatment which may reduce symptoms of breathlessness. However, we can’t promise that everyone will experience clear benefits. The results of the study will help us to understand how to design a future larger study to test this new treatment for people with asthma.
Who is organizing and funding the research?
This study is being led (and sponsored) by the University of Salford. The study has been funded by the National Institute for Health Research, which is part of the NHS.
How will we use information about you?
We will need to use information from you for this research project. This information will include your initials, NHS number, name and contact details. People will use this information to do the research or to check your records to make sure that the research is being done properly. People who do not need to know who you are will not be able to see your name or contact details. Your data will have a code number instead.The University of Salfordis responsible for looking after your information. We will share your information related to this research project with other universities and NHS organisations. We will keep all information about you safe and secure by ensuring it is saved on password protected computers within encrypted files. Your data will not be shared outside the UK.
How will we use information about you after the study ends?
Once we have finished the study, we will keep some of the data so we can check the results. We will write our reports in a way that no-one can work out that you took part in the study. We will keep your study data for a maximum of 3 of years. The study data will then be fully anonymised and securely archived or destroyed. We are happy to send each participant in the study a summary of the results. Please indicate on the consent form if you would like to receive this summary and confirm that you are happy for us to retain your contact information for 3 years to allow us to send this information to you. If you take part in an interview, then we may use anonymised quotes when writing up our results. No identifiable data will be kept after the end of the study (apart from contact details if you would like a summary of the results).
What are your choices about how your information is used?
You can stop being part of the study at any time, without giving a reason, but we will keep information about you that we already have. You have the right to ask us to access, remove, change or delete data we hold about you for the purposes of the study. You can also object to our processing of your data. We might not always be able to do this if it means we cannot use your data to do the research. If so, we will tell you why we cannot do this. Note that, if you do decide to withdraw, you would no longer receive the treatment. If you want to withdraw, please notify the study representative listed in the “Further information and contact details” section below.
Where can you find out more about how your information is used?
You can find out more about how we use your information at https://www.salford.ac.uk/privacy or by asking one of the research team.
What if there is a problem?
The university has insurance to cover against any harm to you which may occur whilst you are taking part in these tests. However, if you decide to take legal action, you may have to pay for this. If you wish to complain, or have any concerns about any aspect of the way you have been approached or treated during the course of this study, you can contact the project supervisor Prof Stephen Preece (email: s.preece@salford.ac.uk) and if you are not happy you may then contact Dr Katy Szczepura, Ethics Chair, Allerton Building, University of Salford, M5 4WT via email: K.Szczepura@salford.ac.uk.
Further information and contact details:
If you require more information about the study, want to participate, or if you are already participating and want to withdraw, please contact:
- Email: a.m.handley@salford.ac.uk
- Tel: +44 (0) 161 295 6758
We appreciate your interest in this study and hope to welcome you at the School of Health and Society, University of Salford. If you are interested in taking part in this study then please get in touch. Contact details and the online form can be found on our BREATH website.