Care Across the Lifecourse
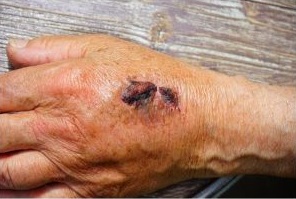
Care across the Lifecourse encompasses research across various stages of life or population groups and covers multiple disciplines across health and social care. For example, it seeks to develop the evidence base of fundamental areas of nursing care including tissue viability, wound care, the care of non-ventilated COVID patients, mortality and morbidity associated and the care of older women with urinary incontinence, frailty and cardiothoracic surgery. Professional learning is key to providing high quality care and forms an important area of work for this theme including, for example a project which establishes and assesses the impact of an interprofessional student training environment for university students from a range of professional health and social care programmes working in the care home sector. Funding associated with this group originates from the Medical Research Council (MRC), National Institute for Health Research (NIHR) and NHS Health Education England.